Abstract
Salivary agglutinin (SAG), also known as gp340 or SALSA, is a glycoprotein encoded by the Deleted in Malignant Brain Tumours 1 gene and is abundantly present in human saliva. SAG aggregates bacteria and viruses, thereby promoting their clearance from the oral cavity. The mucosa lining the oral cavity contains dendritic cells (DC) and Langerhans cells (LC), which express the C-type lectin receptors (CLR) DC-SIGN and Langerin, respectively. Both DC-SIGN and Langerin recognise mannose and fucose carbohydrate structures on pathogens and self-glycoproteins to regulate immunity and homeostasis. The purpose of this study was to investigate whether SAG interacts with these CLR and whether this interferes with the binding to oral pathogens. We show that whole parotid saliva and SAG, when coated to microplates, strongly interact with DC-SIGN and Langerin, probably via mannose and fucose structures. Also, primary human DC and LC bind parotid saliva and SAG via DC-SIGN and Langerin, respectively. Furthermore, SAG binding to DC-SIGN or Langerin prevented binding to the micro-organisms Candida albicans and Escherichia coli which express mannose and fucose-containing glycan structures. Thus, binding of saliva glycoprotein SAG to DC-SIGN and Langerin may inhibit pathogen-DC/LC interactions, and could prove to be a new immunomodulatory mechanism of SAG.
Introduction
The oral mucosa lines the oral cavity and protects the host against invading pathogens. It contains an elaborate immune system [1], which is thought to be protolerogenic as the oral mucosa remains in homeostasis despite the heavy antigenic load from food and micro-organisms [2]. Along the entire oral mucosa, Langerhans cells (LC) are present in the mucosal epithelium and dendritic cells (DC) in the lamina propria [3,4]. Oral DC and LC are of major importance in the induction of immunity and tolerance. As in other tissues, oral DC and LC are able to capture antigens and migrate to the draining lymph nodes for the induction of T cell responses [1].
DC and LC express a variety of receptors for capturing pathogens and initiating immune responses, including C-type lectin receptors (CLR). CLR are a family of carbohydrate receptors that recognise a variety of glycan moieties via their carbohydrate recognition domain(s) [5]. They are internalisation receptors for pathogens, generally resulting in the initiation of an immune response. Nevertheless, they can also interact with self-glycoproteins, thereby exerting important functions in immune modulation and immune homeostasis. The CLR expression pattern is highly specific for DC and LC, with DC-SIGN expressed by DC and Langerin expressed by LC [3,4]. Both receptors show overlap in their binding specificities to mannosylated moieties, but their binding preference to fucose structures differs [6,7,8]. DC-SIGN binds to terminal mono- and di-fucose structures, whereas Langerin only binds to di-fucosylated structures. These different fucose structures on self-glycoproteins are genetically determined. Depending on the function of the α1,2-fucosyltransferase (FUT)2, encoded by the Se gene, glycoproteins express antigens with 1-terminal or 2-terminal fucoses [9]. Consequently, individuals have been named secretors when having a functional FUT2, thus expressing the blood group antigens, Lewis (Le)b and Ley with 2-terminal fucoses in addition to Lea and Lex, containing a 1-terminal fucose. Alternatively, non-secretors only express the mono-fucosylated Lea and Lex.
Human saliva has anti-viral, anti-bacterial and anti-fungal activity [10,11]. It contains several host defence factors and specific bacterial-binding proteins, such as mucins, histatins, β-defensins, lysozyme, secretory IgA and salivary agglutinin (SAG) [12]. SAG, also known as gp340 or SALSA, is encoded by the Deleted in Malignant Brain Tumours 1 (DMBT1) gene. It is a glycoprotein that is present in tears and lung fluid and on mucosal surfaces along the gastrointestinal tract, and is highly sialylated and fucosylated [13]. SAG is a high-molecular-weight glycoprotein of 340 kDa belonging to the scavenger receptor cysteine-rich superfamily. Via both protein and carbohydrate ligand-binding domains SAG mediates adhesion and aggregation to pathogen-associated molecular patterns of Gram-negative and Gram-positive bacteria such as Streptococcus mutans, Streptococcus gordonii, Staphylococcus aureus, Helicobacter pylori, Escherichia coli and Lactobacillus acidophilus, the influenza A viruses and HIV, thereby promoting their clearance from the oral cavity [13]. Next to pathogenic patterns, SAG displays binding to a variety of endogenous molecules that play a role in innate immunity, such as secretory IgA and collectin SP-A and SP-D, and complement factors C1q and mannose-binding lectin (MBL) [13,14,15].
Despite these described innate immune functions of SAG, the direct influence on immune cell function by this glycoprotein has not yet been addressed. The specificity of CLR for self-glycoproteins has been considered important for the maintenance of immune homeostasis. The localisation of CLR-expressing DC and LC at mucosal surfaces suggests a role for salivary glycoproteins in the modulation of immune responses and/or mucosal homeostasis. Similarly, it has been described that human milk glycoprotein MUC1 blocks the HIV-1 to DC interaction, thus preventing HIV transmission to T cells, via the interaction of the Lex moiety on MUC1 to DC-SIGN [16,17]. Here, we demonstrate that SAG, a glycosylated protein abundantly present in human saliva, specifically binds DC-SIGN and Langerin on DC and LC, and effectively inhibits DC-SIGN- and Langerin-mediated binding to oral micro-organisms.
Materials and Methods
Immunohistochemistry
Human adult gingival tissue was obtained from 10 patients undergoing oral implantology (5 healthy) or wisdom tooth extraction (5 inflamed) and used in an anonymous fashion, in accordance with the ‘Code for Proper Use of Human Tissues' as formulated by the Dutch Federation of Medical Scientific Organisations (www.fmwv.nl) and following procedures approved by the institutional review board of the VU University Medical Center.
For immunohistochemical staining of gingiva biopsies, the samples were embedded in paraffin. Vertical 5-μm sections were cut from 10 different donors, sections were de-paraffinised and rehydrated in preparation for immunohistochemical analysis. Immunohistochemical procedures were performed as previously described [18]. In brief, antigen retrieval was performed using citrate buffer and, after cooling, was incubated overnight at room temperature with primary monoclonal antibodies directed against either DC-SIGN (clone DCN46, BD Pharmingen, San Diego, Calif., USA), CD14 (clone TÜK4, Dako, Glostrup, Denmark), Langerin (clone 12D6, NCL-Langerin, Leica, Newcastle upon Tyne, UK) or CD1a (clone MTB1, MONX10315, Monosan, Uden, The Netherlands). After washing in PBS for 5 min, sections were incubated for another 30 min with human anti-mouse conjugated to HRP (Envision, DakoCytomation). After washing with PBS, the slides were incubated for 10 min with 3-amino-9-ethylcarbazole (Invitrogen, San Francisco, Calif., USA) as the chromogen. All sections were counter-stained with haematoxylin (Sigma Chemical Co., St. Louis, Mo., USA). Negative controls were prepared by omitting the primary antibody and substituting an isotype control antibody. The sections were embedded in Aquatex® (Merck, Darmstadt, Germany).
Parotid Saliva Collection and SAG Enrichment
Parotid saliva was collected from healthy volunteers with a Lashley cup, under the stimulation of menthol chewing gum. The samples were centrifuged (for 5 min at 4,000 g) and debris was discarded. Further enrichment of SAG was conducted as described previously [19]. Purity was checked by periodic acid-Schiff (PAS)-stained gel (online suppl. fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000443016). Protein content of SAG-enriched samples ranged from 17 to 150 μg/ml, determined by means of a BCA assay (Thermo Scientific, Rockford, Ill., USA).
Micro-Organisms and Growth Conditions
Candida albicans315 (ATCC 10231) was cultured on Sabouraud dextrose agar plates under aerobic conditions at 30°C. Streptococcus salivarius (HB) and E. coli (BL21) were maintained on tryptic soy agar plates under aerobic conditions at 37°C. Colonies were inoculated overnight in Sabouraud dextrose broth for C. albicans (aerobically at 30°C), and tryptic soy broth for S. salivarius and E. coli(aerobically at 37°C). We used overnight cultures (stationary phase of the micro-organisms), since most micro-organisms in the oral cavity are in the biofilm and phenotypically similar to stationary-phase cultures. Yeasts and bacteria were harvested by centrifugation (for 5 min at 5,000 g), washed in PBS and then resuspended in PBS to an optical density (OD) at 600 nm of 1.0. Next, suspensions of 109 CFU/ml were coated in 96-well plates (Greiner Microlon, Greiner Bio-One, Frickenhausen, Germany) overnight at 4°C. Cells were fixed with methanol for 15 min at room temperature, followed by washes with PBS.
Lectin ELISA
Immuno Maxisorp plates (Nunc, Roskilde, Denmark) were either coated with parotid saliva or enriched SAG samples (in dilutions and concentrations as stated) in 0.05 M Na2CO3 (pH 9.8) buffer overnight at 4°C. To avoid non-specific binding, the plates were blocked in Tris-sodium buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1 mM CaCl2 and 2 mM MgCl2) containing 1% bovine serum albumin (BSA; fraction V, fatty acid-free, Calbiochem, San Diego, Calif., USA). For C-type lectin binding, we used chimeric constructs that consist of the extracellular domain of the CLR fused to the Fc portion of human IgG1. Plates were washed in Tris-sodium with 0.05% Tween and incubated with DC-SIGN-Fc or Langerin-Fc [20] (2 μg/ml) for 2 h at room temperature, in the absence or presence of the calcium-chelator EGTA (10 mM). After washing, binding was detected by peroxidase-labelled goat anti-human IgG/Fcγ-specific F(ab′)2 (Jackson ImmunoResearch Europe, Newmarket, UK). For plant lectin binding, plates were washed and incubated with the biotinylated plant lectins, Helix pomatia agglutinin (HPA; Sigma-Aldrich), Ulex europaeus agglutinin (UEA)-I, Lotus tetragonolobus agglutinin (LTA), Pisum sativum agglutinin (PSA), Narcissus pseudonarcissus agglutinin (NPA) and Galanthus nivalis agglutinin (GNA) (5 μg/ml; Vector Laboratories) for 2 h at room temperature, and binding was detected by peroxidase-labelled streptavidin (Invitrogen/Life Technologies). The reaction was developed in 100 μg/ml 3,3′-5,5′-tetramethylbenzidine (TMB) as a substrate (Sigma-Aldrich) and OD was measured by a microplate absorbance spectrophotometer (Biorad) at 450 nm.
Binding to coated oral micro-organisms was analysed with DC-SIGN-Fc (2 μg/ml) and Langerin-Fc (1 μg/ml) in the presence or absence of EGTA (10 mM) or mannan (1 mg/ml), in order to determine CLR-specific binding, or in the presence of SAG samples (in 1:10 dilution) pre-incubated for 1 h at room temperature with DC-SIGN-Fc and Langerin-Fc to block CLR interaction with oral micro-organisms.
Cells
Wild-type Raji, Raji-DC-SIGN and Raji-Langerin cells were cultured in RPMI 1640 medium (Invitrogen, Paisley, UK) supplemented with 10% fetal calf serum (FCS), 50 U/ml penicillin, 50 μg/ml streptomycin and 2 mM glutamine (all from Lonza, Verviers, Belgium).
Human immature DC were generated from monocytes isolated from buffy coats of healthy donors (Sanquin, Amsterdam, The Netherlands), obtained after informed consent. Buffy coats were mixed with PBS containing 0.45% citrate, and peripheral blood mononuclear cells (PBMC) were isolated by a Ficoll gradient (Lymfoprep; Axis-Shield PoC AS, Oslo, Norway). PBMC were washed and monocytes isolated by a Percoll gradient (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Monocytes were cultured for 5-6 days in RPMI 1640 medium (Invitrogen) supplemented as above, in the presence of recombinant human IL-4 and GM-CSF (500 and 800 U/ml, respectively; Immunotools, Friesoythe, Germany).
Primary human LC were isolated from skin explants derived from abdominal resections from healthy donors (Bergman Clinics, Bilthoven, The Netherlands) that were obtained with informed consent within 24 h after surgery as described previously [21]. Briefly, 5-mm-thick slices of skin, containing the epidermis and dermis, were cut using a dermatome. The slices were incubated in Dispase II (1 mg/ml, Roche Diagnostics) in IMDM (Invitrogen) supplemented with 10% FCS, 50 U/ml penicillin, 50 μg/ml streptomycin (Lonza) and 10 μg/ml gentamycin (Invitrogen) overnight at 4°C, followed by the mechanical separation of dermis and epidermis using tweezers. The epidermis was washed in PBS and cultured for 2 days in IMDM supplemented with 10% FCS, 50 U/ml penicillin, 50 μg/ml streptomycin, 10 μg/ml gentamycin and 800 U/ml GM-CSF (Immunotools), to allow migration of the LC. After 2 days, migrated cells were harvested and layered on a Ficoll gradient (Axis-Shield PoC AS) to further purify the cells (routinely >85% purity is reached).
Cell Adhesion Assay
Immuno Maxisorp plates (NUNC) were coated with enriched SAG samples (in 1:100 dilution) in 0.05 M of Na2CO3 (pH 9.8) buffer overnight at 4°C. After washing with trisodium buffer, cells labelled with calcein-acetoxymethyl (Invitrogen, Eugene, Oreg., USA) were added in the presence or absence of EGTA (5 mM) or 10 μg/ml neutralising antibodies against DC-SIGN (AZN-D1) [22] or Langerin (10E2) [21] and incubated for 90 min at 37°C. Non-adherent cells were gently washed away and adherent cells were lysed in 50 mM Tris/0.1% SDS. Fluorescence was quantified on a Fluostar spectrofluorimeter (BMG Labtech) at 485/520 nm and the percentage of cell-binding was calculated relative to the fluorescence of the total cell input without washing away (100% control).
Flow Cytometry
Phenotypical analysis of primary DC and LC was performed by flow cytometry. Cells were washed in PBS supplemented with 1% BSA and 0.02% NaN3, and incubated for 30 min at 4°C in the presence of appropriate dilutions of fluorescent-conjugated monoclonal antibodies to DC-SIGN (R&D Systems, Minneapolis, Minn., USA) and Langerin (CD207; Beckman Coulter, Marseille, France). The cells were subsequently analysed using FACSCalibur (Becton Dickinson, San Jose, Calif., Oreg., USA) and FlowJo software (Tree Star, Ashland, Oreg., USA).
Statistical Analysis
Results were analysed using either a one-way ANOVA followed by Dunnett's post hoc test or a two-way ANOVA followed by Bonferroni's post hoc test, using GraphPad Prism software v5.01 (San Diego, Calif., USA). Values were considered to be significantly different when p < 0.05.
Results
The Human DC- and LC-Specific CLR DC-SIGN and Langerin Are Present in the Oral Mucosa
To establish the presence of DC and LC and their expressed CLR in the oral mucosa, biopsies from healthy and inflamed gingiva tissue were stained for DC-SIGN and Langerin, respectively. DC-SIGN-positive cells were detected in the lamina propria (fig. 1). Upon inflammation, more DC-SIGN-positive cells were detected, indicating recruitment of DC to inflamed tissue or upregulation of the receptor. DC in oral lamina propria express CD14, which we detected mainly upon inflammation. Langerin-positive cells were found in the epithelium of inflamed tissue, as well as CD1a-positive cells, both being markers for LC. Thus, DC-SIGN and CD14 expression demonstrates the presence of DC in the lamina propria, and Langerin and CD1a expression demonstrates the presence of LC in the epithelium of oral gingival mucosa. The expression of these markers was elevated in inflamed tissue, as was previously described [1].
Expression of DC-SIGN and Langerin in oral mucosa gingival tissue: staining of DC in lamina propria with DC-SIGN and CD14, and of LC in epithelium with Langerin and CD1a. Gingiva biopsies of healthy and inflamed tissue. ×200. Representative photographs of tissue from 10 different donors (5 healthy and 5 inflamed).
Expression of DC-SIGN and Langerin in oral mucosa gingival tissue: staining of DC in lamina propria with DC-SIGN and CD14, and of LC in epithelium with Langerin and CD1a. Gingiva biopsies of healthy and inflamed tissue. ×200. Representative photographs of tissue from 10 different donors (5 healthy and 5 inflamed).
Human Saliva Contains Glycoproteins That Interact with the DC- and LC-Specific CLR DC-SIGN and Langerin
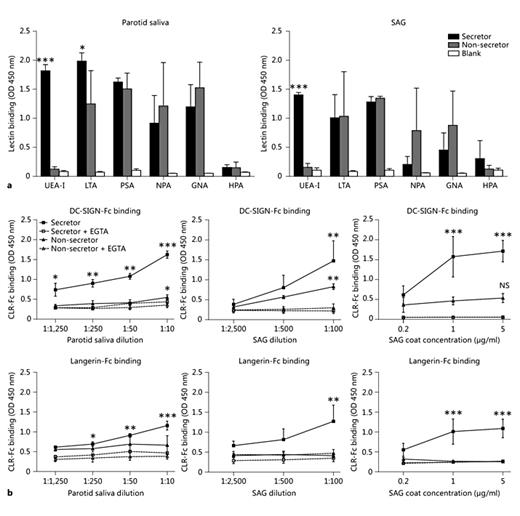
To investigate the presence of DC-SIGN- and Langerin-binding glycoproteins in human saliva, the interaction of DC-SIGN-Fc and Langerin-Fc with human parotid saliva was tested by ELISA. DC-SIGN-Fc- and Langerin-Fc-binding could be influenced by the secretor status of the donor, as secretors express both mono- and difucose structures whereas non-secretors only express mono-fucosylated structures and thus vary in Le blood group antigens present on the glycoproteins in saliva. Therefore, we determined the secretor status of the saliva donors with UEA-I, a plant lectin recognising α1-2 fucose [23,24,25,26]. This glycan structure is present in the Lewisb and Lewisy blood group antigens presented by secretors only. We used the UEA-1 lectin next to antibodies against Lewisb and Lewisy to demonstrate the secretor status of the saliva donors (table 1).
We determined the presence of fucose and mannose moieties in parotid saliva and on SAG with several plant lectins with known binding specificities, as these sugar structures are ligands for DC-SIGN and Langerin. As expected, α1-2 fucose (UEA-I) is present only in the parotid saliva and SAG of secretor donors, not non-secretor donors (fig. 2a; table 1). α1-3/4 fucose (LTA) is present in both secretor and non-secretor donors; it can be a ligand for DC-SIGN, and for Langerin when difucosylated. In addition, mannose-type structures are present in the parotid saliva and on SAG (PSA, NPA and GNA). In contrast, there were no α-N-acetylgalactosamine (αGalNAc) sugar structures present in the parotid saliva or SAG. αGalNAc is a ligand for the DC and macrophage CLR, macrophage galactose lectin [27]. Accordingly, we observed no binding of macrophage galactose lectin-Fc to the parotid saliva or SAG of both secretor and non-secretor donors (data not shown).
Saliva contains glycoproteins that interact with human DC- and LC-expressed lectins. a Plant lectin binding to parotid saliva and glycoprotein SAG of secretor and non-secretor donors to determine sugar structures present in saliva. Data are shown as mean ± SEM of 3 independent donors. b We determined the presence of glycoproteins that contain ligands for DC-SIGN and Langerin in human saliva by ELISA using DC-SIGN-Fc and Langerin-Fc constructs. Incubation was performed in the absence or presence of EGTA. Whole parotid saliva or SAG glycoprotein of secretor and non-secretor donors was coated in indicated dilutions/concentrations. Data are shown as mean ± SEM of 3 or 4 independent donors. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Saliva contains glycoproteins that interact with human DC- and LC-expressed lectins. a Plant lectin binding to parotid saliva and glycoprotein SAG of secretor and non-secretor donors to determine sugar structures present in saliva. Data are shown as mean ± SEM of 3 independent donors. b We determined the presence of glycoproteins that contain ligands for DC-SIGN and Langerin in human saliva by ELISA using DC-SIGN-Fc and Langerin-Fc constructs. Incubation was performed in the absence or presence of EGTA. Whole parotid saliva or SAG glycoprotein of secretor and non-secretor donors was coated in indicated dilutions/concentrations. Data are shown as mean ± SEM of 3 or 4 independent donors. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Next, we analysed DC-SIGN-Fc- and Langerin-Fc-binding to parotid saliva, and observed that DC-SIGN and Langerin both strongly interacted with parotid saliva from donors with secretor status, as was expected with the presence of ligands for both DC-SIGN and Langerin (fig. 2b). This interaction was inhibited by the calcium chelator, EGTA, demonstrating the specificity of the glycan-lectin interaction. Only DC-SIGN interacted with saliva from non-secretor donors, who do not express Langerin ligand α1-2 fucose. Although mannose structures are present in the saliva of non-secretor donors, we did not detect Langerin binding to non-secretor samples. We further analysed CLR binding to SAG, which is a major glycoprotein in human parotid saliva [28]. Again, there was a strong interaction of DC-SIGN with SAG from secretor and non-secretor donors, whereas Langerin only interacted with SAG from secretor donors (fig. 2b). Thus, mannose- and fucose-containing glycoproteins, including SAG, are present in human saliva, and they bind to human DC-SIGN and Langerin.
DC and LC Bind to Saliva and SAG Glycoprotein via DC-SIGN and Langerin
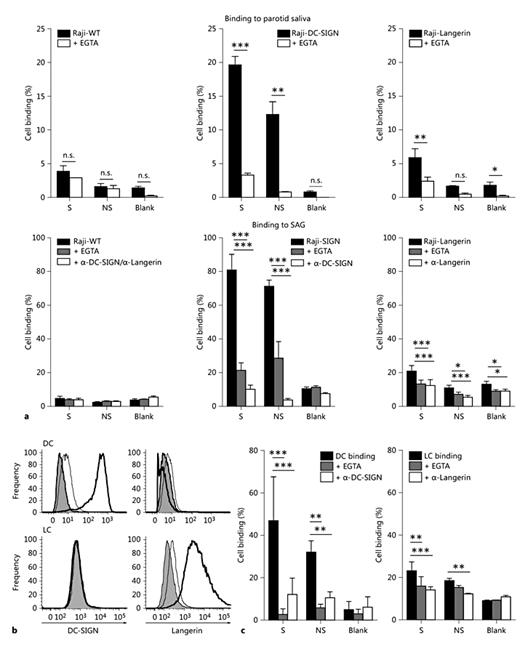
To determine the interaction of biologically functional human CLR to parotid saliva and SAG, we first used DC-SIGN- and Langerin-transfected Raji cells in a cell adhesion assay. Wild-type Raji cells did not specifically bind to saliva or SAG (fig. 3a), while Raji-DC-SIGN cells strongly adhered to the parotid saliva and SAG of secretor and non-secretor donors in a DC-SIGN-dependent manner. There was a weaker, but significant, Langerin-specific interaction of Raji-Langerin cells with the parotid saliva and SAG of secretor donors. However, Langerin binding was lower than the DC-SIGN binding to parotid saliva and SAG, which correlated with the lower expression of Langerin on Raji-Langerin cells compared to a high expression of DC-SIGN on Raji-DC-SIGN cells (data not shown). There was no specific interaction of Raji-Langerin with the parotid saliva and SAG of non-secretor donors compared to the blank control.
Primary DC and LC bind to the glycoprotein SAG. Cell-binding assay with fluorescently labelled Raji cells (a) or DC and LC (c) to coated parotid saliva (1:10 dilution) or SAG (1:100 dilution), with or without EGTA or blocking antibodies against DC-SIGN and Langerin. Data are shown as mean ± SEM of 3 independent experiments. The percentage of cell binding was calculated relative to the total fluorescence of the specific start cell populations. Total fluorescence (100% control) of the different cell populations ranged from 33,548 to 38,874, with an average of 36,600 ± 1,946. b Expression of DC-SIGN and Langerin on monocyte-derived DC and epidermal LC was determined by flow cytometry. Secondary antibody control: gray histograms, isotype control: thin line, CLR staining: thick line. Blank = No donor; NS = non-secretor donor; S = secretor donor. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Primary DC and LC bind to the glycoprotein SAG. Cell-binding assay with fluorescently labelled Raji cells (a) or DC and LC (c) to coated parotid saliva (1:10 dilution) or SAG (1:100 dilution), with or without EGTA or blocking antibodies against DC-SIGN and Langerin. Data are shown as mean ± SEM of 3 independent experiments. The percentage of cell binding was calculated relative to the total fluorescence of the specific start cell populations. Total fluorescence (100% control) of the different cell populations ranged from 33,548 to 38,874, with an average of 36,600 ± 1,946. b Expression of DC-SIGN and Langerin on monocyte-derived DC and epidermal LC was determined by flow cytometry. Secondary antibody control: gray histograms, isotype control: thin line, CLR staining: thick line. Blank = No donor; NS = non-secretor donor; S = secretor donor. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
Next, we used primary human monocyte-derived DC and human epidermal LC for binding to SAG in a cell adhesion assay. We confirmed the expression of DC-SIGN on DC only, whereas Langerin was expressed only on LC (fig. 3b). Again, there was significant and strong binding of DC to SAG of both secretor and non-secretor donors in a DC-SIGN-specific manner. Also LC bound to the SAG of secretor donors and non-secretor donors in a Langerin-dependent manner, although to a lesser extent than DC. These data show that primary human DC and LC bind to glycoprotein SAG via their respective CLR.
DC-SIGN and Langerin Bind to Oral Micro-Organisms
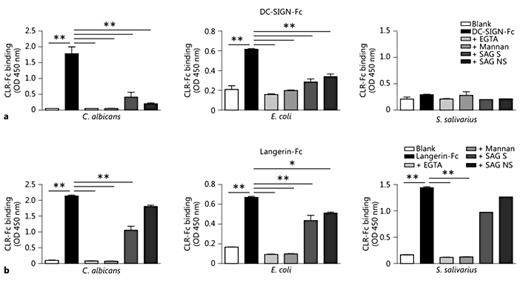
We hypothesised that the interaction of DC-SIGN and Langerin with glycoprotein SAG may inhibit the interaction of these CLR with oral pathogens, and thereby maintain oral homeostasis. For this, we first investigated whether DC-SIGN and Langerin bind to the yeast C. albicans, a common oral pathogen responsible for oral candidiasis. We also investigated the binding to an oral commensal, S. salivarius, which may act as an opportunistic pathogen, and to E. coli, heavily present in the gastrointestinal tract. Both DC-SIGN-Fc and Langerin-Fc bound strongly to C. albicans (fig. 4a). Langerin also bound specifically to E. coli and S. salivarius, whereas DC-SIGN-Fc showed much weaker binding to these micro-organisms. With the plant lectin ELISA, we confirmed the presence of different fucose (UEA-I and LTA) and mannose (PSA, GNA and NPA) structures on these micro-organisms (fig. 4b), thereby confirming the presence of DC-SIGN- and Langerin-binding carbohydrate ligands. Thus, DC-SIGN and Langerin bind to the micro-organisms C. albicans, E. coli and S. salivarius.
DC-SIGN and Langerin bind to oral micro-organisms. a We determined binding of DC-SIGN and Langerin to oral micro-organisms by ELISA using DC-SIGN-Fc and Langerin-Fc constructs. Micro-organisms were coated onto ELISA plates (109 CFU/ml) and incubation was performed in the absence or presence of EGTA. Data are shown as mean ± SEM of 5 independent experiments. b Plant lectin binding to oral micro-organisms to determine the sugar structures present on the micro-organisms. Data of 1 experiment is shown. *** p ≤ 0.001.
DC-SIGN and Langerin bind to oral micro-organisms. a We determined binding of DC-SIGN and Langerin to oral micro-organisms by ELISA using DC-SIGN-Fc and Langerin-Fc constructs. Micro-organisms were coated onto ELISA plates (109 CFU/ml) and incubation was performed in the absence or presence of EGTA. Data are shown as mean ± SEM of 5 independent experiments. b Plant lectin binding to oral micro-organisms to determine the sugar structures present on the micro-organisms. Data of 1 experiment is shown. *** p ≤ 0.001.
SAG Prevents Interaction between Oral Micro-Organisms and the CLR DC-SIGN and Langerin
To test our hypothesis that SAG interaction with CLR inhibits the interaction with micro-organisms, we analysed the binding of DC-SIGN-Fc and Langerin-Fc to different micro-organisms in the presence of the SAG of secretor and non-secretor donors. The binding of DC-SIGN-Fc and Langerin-Fc to C. albicans and E. coli could be blocked with EGTA and mannan, demonstrating CLR-specific binding (fig. 5a, b). The addition of SAG significantly inhibited the interaction of DC-SIGN-Fc and Langerin-Fc with C. albicans and E. coli. DC-SIGN-Fc showed no significant binding to S. salivarius (fig. 5a). Langerin-Fc bound strongly to S. salivarius, and the SAG of secretor donors showed a trend of inhibiting this interaction (fig. 5b). These data demonstrate the potential of SAG glycoprotein to inhibit the interaction of CLR with pathogens and commensals present in the oral cavity.
DC-SIGN and Langerin binding to oral micro-organisms is inhibited with SAG. We determined binding of DC-SIGN and Langerin to oral micro-organisms by ELISA using DC-SIGN-Fc and Langerin-Fc constructs. Micro-organisms were coated onto ELISA plates and incubation was performed in absence or presence of EGTA, mannan or SAG (1:10 dilution). Data are shown as mean ± SEM of 3 (C. albicans and E. coli) or 1 (S. salivarius) independent SAG donors. Blank = No donor; NS = non-secretor donor; S = secretor donor. * p ≤ 0.05, ** p ≤ 0.01.
DC-SIGN and Langerin binding to oral micro-organisms is inhibited with SAG. We determined binding of DC-SIGN and Langerin to oral micro-organisms by ELISA using DC-SIGN-Fc and Langerin-Fc constructs. Micro-organisms were coated onto ELISA plates and incubation was performed in absence or presence of EGTA, mannan or SAG (1:10 dilution). Data are shown as mean ± SEM of 3 (C. albicans and E. coli) or 1 (S. salivarius) independent SAG donors. Blank = No donor; NS = non-secretor donor; S = secretor donor. * p ≤ 0.05, ** p ≤ 0.01.
Discussion
In this study, we demonstrate that the highly glycosylated agglutinin SAG from human saliva interacted with DC-SIGN and Langerin on human DC and LC, respectively. More importantly, SAG efficiently blocked the interaction of oral pathogens and commensals with DC-SIGN and Langerin. It has previously been demonstrated that the glycoprotein MUC1, an epithelial mucin that is abundantly present in human milk, inhibits HIV-1 transmission [16]. Saeland et al. [16 ]showed that MUC1 binds to DC-SIGN, most likely via repetitive units of Lex, and that the transmission of HIV-1 from DC to T cells is prevented by MUC1 through competition with HIV-1 for DC-SIGN binding. We here demonstrate that various oral micro-organisms bind to DC-SIGN and Langerin. Except for the binding of DC-SIGN and Langerin to C. albicans and of DC-SIGN to E. coli [29,30,31,32], the interaction of Langerin with E. coli and S. salivarius has not been reported before. SAG is extensively glycosylated, containing numerous N-linked and O-linked glycosylation sites, and with carbohydrates representing as much as 25-40% of the molecular weight [13]. We confirm that SAG has various mannose and fucose-containing structures, which are ligands for DC-SIGN and Langerin, and may be responsible for the competition with pathogens in CLR binding. SAG indeed binds to DC-SIGN and Langerin and interferes with the binding of these CLR to oral pathogens. It is for future research to define the exact glycan structures responsible for the interaction of SAG with DC-SIGN and Langerin. Therefore, SAG may play a role in the regulation of immune responses by DC and LC present in the oral mucosa.
We provide evidence that DC- and LC-expressed DC-SIGN and Langerin are new lectin-binding partners for SAG. It was previously shown that SAG binds to MBL [15,33], which is one of the recognition molecules of the lectin pathway of the complement system that recognises carbohydrate structures on pathogens and altered-self cells [34]. The fucose moieties present on SAG are critical for binding to MBL, and this binding activates the lectin pathway of the complement system [19,33]. Besides MBL, another binding partner of SAG is C1q, the recognition molecule of the classical pathway of the complement system [14]. Although SAG is secreted in the saliva, and C1q and MBL are components of serum, these two fluids may mix in the oral cavity under conditions of oral inflammation, e.g. in periodontal disease or mechanical damage of the mucosa, thus enabling local complement activation [35]. The binding of SAG to complement factors MBL and C1q and the subsequent complement activation demonstrate that, besides an agglutinin function, SAG has additional roles in clearing micro-organisms via the complement system. Moreover, an interplay between CLR and the complement system has been described for murine SIGN-R1, the mouse homologue of DC-SIGN, expressed by macrophages. SIGN-R1 directly binds to C1q, and thereby assembles the C3 convertase for C3 deposition on micro-organisms [36] for regulating the complement fixation pathway to fight pathogen infections. Together, these and our data suggest that there is a specific interplay between different defence mechanisms, i.e. cellular lectins with complement factors and salivary glycoproteins, against invading micro-organisms.
Glycosylation of proteins is controlled by genetically encoded glycosyltransferases [37], whereby the secretor status determines the presence of certain Le blood group antigens on SAG [38]. Secretors have SAG containing Lea, Leb, Lex and Ley epitopes, whereas SAG from non-secretors displays only Lea and Lex antigens. We here show that DC-SIGN and Langerin bind strongly to the parotid saliva and SAG of secretor donors, and that the SAG of secretor donors inhibits the binding to oral pathogens by LC more strongly than the SAG of non-secretors. It remains to be determined whether this difference in carbohydrate components on SAG influences host susceptibility to certain infections. In addition, future studies may investigate the inhibition of CLR binding to oral pathogens using biofilm-extracted micro-organisms. Recently, it was shown that SAG of secretor donors binds better to MBL and shows stronger activation of the complement system, mainly due to the presence of the Leb and/ or Ley antigens on SAG [19]. What the secretor status means for oral conditions is not clear. There are studies showing a higher prevalence of caries, chronic periodontitis and Candida infections in non-secretors [39,40,41,42]. In contrast, other studies suggest that there is no significant difference in Candida carriage or bacterial colonisation between secretors and non-secretors [43,44]. Oral inflammation is limited due to the binding of salivary components to the microbial surface. Adhesion of C. albicans to buccal epithelial cells was inhibited by salivary glycans similar to the glycans on buccal epithelial cells [45]. It remains to be determined if the binding of SAG to receptors on oral DC and LC is an additional anti-inflammatory mechanism of SAG.
In conclusion, this study demonstrates that the salivary glycoprotein SAG is capable of binding to DC-SIGN and Langerin, both expressed in the oral mucosa. The interaction of SAG with these CLR inhibited the binding to the micro-organisms C. albicans and E. coli. This provides the first evidence for a role of glycoprotein SAG in DC and LC function through the interaction with DC-SIGN and Langerin, and suggests the potential role of SAG to block the interaction with oral pathogens via these uptake receptors at the oral mucosa.
Acknowledgements
We would like to thank the personnel and skin donors of the Bergman Clinic in Bilthoven, The Netherlands, for providing the healthy donor skin. This work was funded by a grant from Top Institute Pharma (T1-501) and European Research Council (ERC-Advanced339977).
Disclosure Statement
The authors state no conflict of interest.