Abstract
Background: Tissue recruitment and activation of eosinophils contribute to allergic symptoms by causing airway hyperresponsiveness and inflammation. Shape changes and mediator release in eosinophils may be regulated by mammalian Rho-related guanosine triphosphatases. Of these, Rac2 is essential for F-actin formation as a central process underlying cell motility, exocytosis, and respiratory burst in neutrophils, while the role of Rac2 in eosinophils is unknown.We set out to determine the role of Rac2 in eosinophil mediator release and F-actin-dependent shape change in response to chemotactic stimuli. Methods: Rac2-deficient eosinophils from CD2-IL-5 transgenic mice crossed with rac2 gene knockout animals were examined for their ability to release superoxide through respiratory burst or eosinophil peroxidase by degranulation. Eosinophil shape change and actin polymerization were also assessed by flow cytometry and confocal microscopy following stimulation with eotaxin-2 or platelet-activating factor. Results: Eosinophils from wild-type mice displayed inducible superoxide release, but at a small fraction (4–5%) of human eosinophils. Rac2-deficient eosinophils showed significantly less superoxide release (p < 0.05, 26% less than wild type). Eosinophils lacking Rac2 had diminished degranulation (p < 0.05, 62% less eosinophil peroxidase) and shape changes in response to eotaxin-2 or platelet-activating factor (with 68 and 49% less F-actin formation, respectively; p < 0.02) compared with wild-type cells. Conclusion: These results demonstrate that Rac2 is an important regulator of eosinophil function by contributing to superoxide production, granule protein release, and eosinophil shape change. Our findings suggest that Rho guanosine triphosphatases are key regulators of cellular inflammation in allergy and asthma.
Introduction
Eosinophils are major effector cells in inflammation and accumulate in tissues in allergy and parasitic helminth infection [1]. In mouse models of asthma, eosinophils contribute to mucus production and collagen deposition, leading to lung remodeling [2,3]. Besides classical effector functions, eosinophils are also implicated in immunoregulation [2,4,5,6]. Eosinophil recruitment and accumulation involve transmigration of these cells from blood to tissues in response to chemotactic gradients. Upon their arrival at inflammatory foci, eosinophils are activated to undergo respiratory burst, leading to the generation of anti-microbial products including reactive oxygen species (ROS), de novo synthesized lipid mediators, and stored granule products through exocytotic release. However, the mechanisms that regulate eosinophil shape change as part of cell motility during chemotaxis, as well as how they release mediators, are not well understood.
Rho guanine triphosphatases (GTPases) are monomeric intracellular signaling molecules that regulate actin cytoskeleton remodeling in an evolutionarily conserved pathway from yeast to mammalian cells [7]. Actin remodeling, involving the reversible transformation of globular (G-)actin to filamentous (F-)actin, is a fundamental process in cell motility and exocytosis [8]. Members of the Rho GTPase subfamily include Rac1, Rac2, and Rac3 [7]. Of these, Rac1 and Rac2 share 92% sequence identity, which are functionally interchangeable in their ability to assemble the superoxide-generating NADPH oxidase enzyme complex [9,10,11,12], but have divergent roles in cellular functions [13,14]. Rac2 expression is limited to hematopoietic cells [15] and is involved in a number of activities associated with motility, mediator release, and respiratory burst in neutrophils [14,16]. The leading edge of neutrophils during chemotaxis shows colocalization of Rac2 with F-actin, suggesting that Rac2 is required for the formation of the F-actin cap [17]. A human dominant negative mutation in Rac2 (D57N) generated neutrophils exhibiting deficiencies in chemotaxis and F-actin formation [18]. We showed that primary (azurophilic) granule exocytosis was dependent on Rac2-mediated F-actin remodeling in neutrophils [19,20]. Rac2 plays a dominant role in respiratory burst reactions associated with the activation of the NADPH oxidase enzyme complex, leading to the production of superoxide anion (O2–) and related ROS, including H2O2 and OH–[21]. In human neutrophils, Rac2 is predominantly expressed over Rac1 and preferentially activates the NADPH oxidase enzyme complex [22].
Eosinophils from atopic subjects generate enhanced levels of eosinophil peroxidase (EPO) and ROS [23,24,25], which directly injure airway tissues [26] and together produce further tissue-damaging microbicidal products [23]. We found that Rac was important for O2– release from human eosinophils [27], although the specific Rac isoform involved in this could not be identified due to the lack of specific inhibitors for Rac1 and Rac2. In mouse eosinophils, Rac2 has been shown to be required for eosinophil chemotaxis in response to eotaxin-2 [28]. However, there are no reports describing the specific role of Rac2 in O2– release and degranulation from eosinophils. In addition, little is known regarding mechanisms of activation of mouse eosinophils, which is surprising given their prominence in mouse models of asthma and allergy.
In this study, we set out to determine the specific role of Rac2 in O2– release, degranulation, F-actin formation, and shape change in eosinophils from Rac2-deficient mice. Our data suggest that Rac2 contributes to degranulation and O2– release and has an active role in eosinophil F-actin formation and shape change.
Materials and Methods
Materials
All general chemicals and media, unless otherwise stated, were purchased from Sigma-Aldrich (St. Louis, Mo., USA) or Invitrogen (Carlsbad, Calif., USA). Phorbol myristate acetate (PMA), platelet-activating factor (PAF), and A23187 were prepared at 10 mM in DMSO.
Mice
CD2-IL-5 transgenic mice [29] were housed in specific pathogen-free conditions, as previously described [28,30]. These mice were crossed with Rac2 gene-deficient mice and used as a source of Rac2-deficient eosinophils [28]. Both strains had backgrounds matched to 50% Balb/c, 45% C57Bl/6, and 5% 129 by using a single mating pair of CD2-IL-5/rac2+/– F1 mice to generate F2 offsprings with CD2-IL-5/rac2+/+ and CD2-IL-5/rac2–/– strains, in order to maintain as much genetic homology as possible. Subsequent generations were produced from this original mating pair (i.e. CD2-IL-5/rac2–/– males and females were mated, and CD2-IL-5/rac2+/+ males were also mated; both substrains were viable and fertile). All experiments were conducted on mice 6–12 weeks old. The use of these mice received ethics approval at our institution.
Cell Purification
Spleen cells were isolated from CD2-IL-5 or CD2-IL-5/rac2–/– transgenic mice, which were enriched in eosinophils. Eosinophils were purified by negative selection using anti-Thy1.2 and anti-CD19-conjugated beads (Miltenyi Biotec, Bergisch Gladbach, Germany) [30] and subjected to hypotonic lysis to remove red blood cells. Typically, more eosinophils were obtained from CD2-IL-5/rac2–/– than from CD2-IL-5 mice, which was likely related to eosinophilia observed in rac2–/– mice (see below). Splenocyte preparations from CD2-IL-5 and CD2-IL-5/rac2–/– mice contained 59 ± 1% eosinophils. The purity of eosinophils following immunomagnetic separation was 80–90%. Splenocyte and eosinophil viability was >90% as determined by trypan blue exclusion. Bone marrow neutrophils (BMNs) were prepared from C57Bl/6 wild-type and rac2–/– C57Bl/6 mice by flushing femurs and tibias, then centrifuging cells on Percoll/Hank’s Balanced Salt Solution-BSA and glucose gradients [19]. We obtained 65–70% BMNs with a viability >90% determined by trypan blue exclusion. Human peripheral blood eosinophils (≥97% purity) were prepared as previously described. Blood samples (100 ml) were obtained from mildly atopic subjects exhibiting 5–10% eosinophilia, and who were not receiving oral corticosteroids [27,31]. The use of mouse and human blood samples received approval from our institutional ethics review board.
Immunoblot Analysis
Lysates of splenic eosinophils from CD2-IL-5 and CD2-IL-5/rac2–/– mice or BMNs from wild-type normal C57Bl/6 and rac2–/– mice were subjected to SDS-PAGE and immunoblot analysis [27,32]. Proteins were transferred to 0.2-µm nitrocellulose membranes and blotted with antibody to Rac1 (clone 23A8; Millipore, Etobicoke, Ont., Canada) or Rac2 (Millipore). These were detected using secondary antibodies conjugated to 700 or 800 nm excitation fluorophores, and images were collected on a Li-Cor Odyssey Infrared Imaging System (Lincoln, Nebr., USA).
Measurement of O2– Release
Generation of extracellular O2– from cells in suspension was measured using a cytochrome c reduction assay [27,32]. Briefly, 1 × 107 splenocytes or splenic eosinophils were suspended in 1-ml microcuvettes containing supplemented phosphate-buffered saline, pH 7.4 (with 1.2 mM MgCl2, 5 mM KCl, 0.5 mM CaCl2, 5 mM glucose, and 0.1% BSA) and ferricytochrome c at 25°C. Cell suspensions were blanked at 550 nm in a Beckman DU-640 spectrophotometer (Beckman Instruments, Mississauga, Ont., Canada) before adding PMA. For BMNs, 2 × 106 cells were used for each assay. Superoxide dismutase-inhibitable cytochrome c reduction was calculated using ε = 2.11 × 104M–1 cm–1.
Degranulation Assay
EPO release was determined using a peroxidase assay involving coincubation of peroxidase substrate (o-phenylenediamine, OPD) with cells during stimulation to capture released EPO [33]. Briefly, 2.5 × 104 splenic eosinophils from CD2-IL-5 and CD2-IL-5/rac2–/– mice were added to 96-well flat-bottomed microplates, and 3 µM A23187 (optimized dose from 1–10 µM) in phenol red-free RPMI was added to each well. Control cells were treated with vehicle containing ≤0.5% DMSO. Plates were incubated for 30 min at 37°C, and reactions were terminated by the addition of 100 µl 4 M sulphuric acid. In separate wells, the same quantity of eosinophils was lysed by sonication to obtain a measurement corresponding to 100% EPO cellular content. Plates were read at 492 nm in a spectrophotometric plate reader. The percentage of EPO release was calculated as background-corrected absorbance values for supernatants of stimulated cells divided by corrected absorbance values for lysates.
Actin Polymerization
Polymerization of actin in response to the agonist was assessed using nitrobenzoxadiazole-phallacidin (Molecular Probes, Eugene, Oreg., USA) [34]. Purified eosinophils (2.5 × 106/ml) were suspended in phosphate-buffered saline and stimulated with agonists at 37°C for indicated times. Following stimulation, cells were fixed in 3.7% formaldehyde for 60 min. Lysophosphatidylcholine (100 mg/ml; Sigma-Aldrich) and NBD-phallacidin (3.3 × 10–7M) were added and incubated for 1 h. Cells were analyzed on a FACScalibur (Becton Dickinson, Franklin Lakes, N.J., USA) where fluorescence was proportional to the F-actin content. The relative F-actin content was expressed as the mean channel fluorescence ratio between agonist-treated and -untreated cells. Negative controls for these experiments were cell samples without agonist (done for each genotype), and data were normalized to this as baseline. Positive controls were CD2-IL-5 transgenic eosinophils treated with eotaxin-2 or PAF.
Confocal Image Analysis
Confocal analysis of adherent eosinophils was carried out using rhodamine-phalloidin and DAPI (Molecular Probes) [28]. Splenic eosinophils from CD2-IL-5 and CD2-IL-5/rac2–/– mice were plated at 2–2.5 × 106 cells in 375 µl serum-free Hank’s Balanced Salt Solution into a 8-well chamber slide (Lab-Tek Chambered Coverglass System, Nunc, Rochester, N.Y., USA) and incubated for 1 h at 37°C. PAF (10 nM) and human recombinant eotaxin-2 (CCL24, 10 ng/ml) were prewarmed for 5 min and added to each well for 10 s. Stimulation was terminated by fixation of cells with 2% paraformaldehyde for 20 min. Wells were permeabilized with 0.1% Triton X-100 for 1 min prior to staining. Image collection was carried out on an Olympus FV1000 confocal microscopy system using a Plan Apo 63× objective (1.4 NA; Olympus Canada, Mississauga, Ont., Canada). Eosinophil stimulation with eotaxin-2 or PAF led to a ‘stretched’ morphology in a proportion of cells. Cells were scored for round or stretched morphology in a blinded fashion for genotype and treated samples.
Data Analysis
Data were analyzed by an unpaired t test for groups of 2 experimental conditions. Multiple groups of samples were analyzed by ANOVA with post hoc analysis using Tukey’s multiple comparison test. All experiments were conducted on at least 3 separate occasions. All data with error bars are shown as mean ± SEM.
Results
Rac2 Protein Expression in Splenic Eosinophils
Western blot analysis was carried out on lysates of CD2-IL-5 and CD2-IL-5/rac2–/– splenic eosinophils to confirm Rac1/2 protein expression and gene deletion (fig. 1a). Eosinophils expressed equivalent levels of Rac1 protein in CD2-IL-5 and Rac2–/– samples, suggesting that eosinophil Rac1 was not overexpressed to compensate for Rac2 deficiency, unlike rac2–/– neutrophils which showed an increase in Rac1 expression (fig. 1b) [22,35]. While CD2-IL-5 cells showed significant Rac2 protein expression, CD2-IL-5/rac2–/– lysates showed negligible immunoreactivity for this protein, confirming Rac2 gene deficiency.
Gene deletion of Rac2. a Purified splenic eosinophils (2 × 106 cell equivalents/lane) were isolated from CD2-IL-5 and CD2-IL-5/rac2–/– mice and subjected to Western blot analysis using anti-Rac1 monoclonal or anti-Rac2 polyclonal antibody. Positive control was recombinant baculovirus-derived Rac2 protein, which migrated to a higher molecular weight because of an additional peptide sequence. b Purified BMNs (2 × 106 cell equivalents/lane) were isolated from wild-type C57Bl/6 and rac2–/– mice, and subjected to Western blot analysis as described for a. Rac1 and Rac2 expressions were detected in the same samples analyzed concurrently in separate blots for antibody detection.
Gene deletion of Rac2. a Purified splenic eosinophils (2 × 106 cell equivalents/lane) were isolated from CD2-IL-5 and CD2-IL-5/rac2–/– mice and subjected to Western blot analysis using anti-Rac1 monoclonal or anti-Rac2 polyclonal antibody. Positive control was recombinant baculovirus-derived Rac2 protein, which migrated to a higher molecular weight because of an additional peptide sequence. b Purified BMNs (2 × 106 cell equivalents/lane) were isolated from wild-type C57Bl/6 and rac2–/– mice, and subjected to Western blot analysis as described for a. Rac1 and Rac2 expressions were detected in the same samples analyzed concurrently in separate blots for antibody detection.
Superoxide Release Is Partially Dependent on Rac2 in Splenic Eosinophils
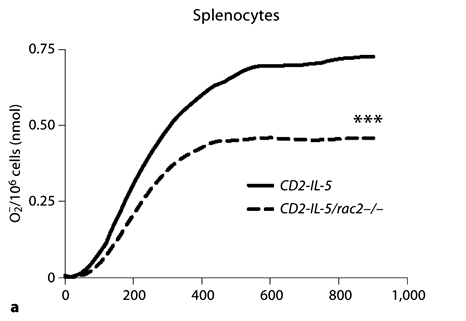
Human and guinea pig eosinophils generate extracellular O2– in response to PMA stimulation in a Rac-dependent manner [27,32,36]. However, there are few reports showing O2– production in mouse eosinophils [37], and its dependency on Rac is unknown. To determine this, we isolated splenocytes from CD2-IL-5 and CD2-IL-5/rac2–/– mice and stimulated them in vitro using a cytochrome c assay that detects only extracellular O2– release. Splenocytes were used as the most abundant source of eosinophils from these mouse strains [28]. Using this assay, splenocytes from CD2-IL-5 mice (1 × 107 cells/measurement) generated approximately 0.7 nmol O2–/106 cells in response to 500 ng/ml PMA (fig. 2a). In contrast, CD2-IL-5/rac2–/– splenocytes generated 37% less O2– than that of CD2-IL-5 mice (p < 0.001). The main cell types found in splenocyte populations were (in decreasing order of abundance) eosinophils, lymphocytes/monocytes, and rarely, neutrophils. Thus, O2– production from splenocytes likely occurred through activation of eosinophils, with negligible contribution from neutrophils.
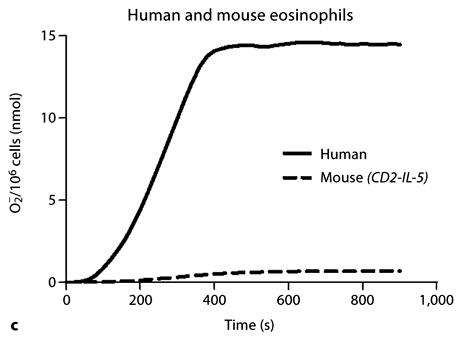
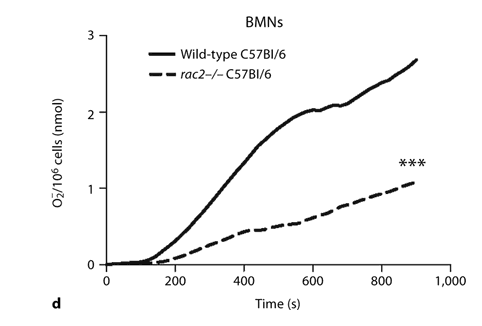
Inducible release of O2– by CD2-IL-5/rac2–/– eosinophils is diminished in comparison with wild-type eosinophils. a Time course of PMA-induced O2– release in splenocytes from CD2- IL-5 and CD2-IL-5/rac2–/– mice (500 ng/ml PMA; n = 9– 10). b PMA-induced O2– release from purified wild-type and CD2-IL-5/rac2–/– eosinophils. c Release of O2– from human and mouse eosinophils in response to PMA (500 ng/ml). d O2– generated from neutrophils from wild-type C57Bl/6 mice compared with rac2–/– mice. b–d n = 3–4. ** p < 0.01, *** p < 0.001.
Inducible release of O2– by CD2-IL-5/rac2–/– eosinophils is diminished in comparison with wild-type eosinophils. a Time course of PMA-induced O2– release in splenocytes from CD2- IL-5 and CD2-IL-5/rac2–/– mice (500 ng/ml PMA; n = 9– 10). b PMA-induced O2– release from purified wild-type and CD2-IL-5/rac2–/– eosinophils. c Release of O2– from human and mouse eosinophils in response to PMA (500 ng/ml). d O2– generated from neutrophils from wild-type C57Bl/6 mice compared with rac2–/– mice. b–d n = 3–4. ** p < 0.01, *** p < 0.001.
To confirm that eosinophils were the main source of O2– release, we purified eosinophils from splenocytes. Eosinophils were subjected to stimulation by 500 ng/ml PMA in the presence of cytochrome c. As shown in figure 2b, purified splenic eosinophils produced similar levels of O2– compared with splenocytes. However, CD2-IL-5/ rac2–/– eosinophils generated significantly less O2– than wild-type eosinophils, although this was not as marked as that observed in splenocytes (26% less, p < 0.01). We also found that mouse eosinophils produced significantly less extracellular O2– compared with human peripheral blood eosinophils (fig. 2c). We tested splenocyte and eosinophil O2– release in response to 5–20 µM f-Met-Leu-Phe, but did not observe a significant release, suggesting that mouse eosinophils lack formyl peptide receptors that trigger O2– release in neutrophils (results not shown).
Since Rac2 is important in inducible O2– release from neutrophils, which are similar cells to eosinophils for their ability to release extracellular O2–, we compared O2– release from BMNs from wild-type control C57Bl/6 and rac2–/– C57Bl/6 mice. Neutrophils could not be purified in sufficient numbers from the bone marrow of IL-5 transgenic mice for comparison with eosinophils from these mice, as neutrophil preparations were contaminated with eosinophil progenitors. As shown in figure 2d, neutrophils from C57Bl/6 mice generated approximately 2–3 nmol O2–/106 cells in response to the same dose of PMA. As expected, rac2–/– neutrophils showed a significantly reduced response (60% less than C57Bl/6 neutrophils). These findings suggest that inducible eosinophil O2– release partially involves Rac2.
Eosinophil Degranulation Is Dependent on Rac2
To determine the role of Rac2 in eosinophil degranulation, we tested responses of eosinophils from CD2-IL-5 and CD2-IL-5/rac2–/– mice to calcium ionophore (A23187). Degranulation was measured by EPO release using an assay developed in our laboratory which employs OPD as the peroxidase-sensitive substrate, incubated directly with cells to detect extracellular EPO immediately after its secretion. Using this approach, 3 µM A23187 was found to induce 35 ± 5% of total cellular EPO release over a baseline release of 9 ± 3% in CD2-IL-5 eosinophils in vehicle (0.5% DMSO)-containing media (n = 8–11, p < 0.01; fig. 3). In contrast, CD2-IL-5/rac2–/– eosinophils were deficient in their EPO release in response to A23187 compared with CD2-IL-5 controls, releasing 21 ± 4% of total EPO over a baseline of 11 ± 3%, which represents a 62% reduction in EPO release compared with CD2-IL-5 cells following subtraction of baseline release in unstimulated cells (p < 0.05; fig. 3). We also tested the effects of eotaxins, IL-5, secretory immunoglobulin-A-coated beads, PMA and PMA plus myristic acid, but found no significant release of EPO from CD2-IL-5 or CD2-IL-5/rac2–/– eosinophils after 4 h of incubation (data not shown). These findings suggest that Rac2 is required for Ca2+-dependent exocytosis from eosinophils.
Deficient calcium-induced exocytosis of CD2-IL-5/rac2–/– eosinophils. In situ release of EPO was determined using OPD as the substrate in samples of 2.5 × 104 freshly prepared eosinophils from CD2-IL-5 and CD2-IL-5/rac2–/– mice (n = 8–11). Cells were stimulated for 30 min in the presence of 3 µm A23187. * p < 0.05, ** p < 0.01.
Deficient calcium-induced exocytosis of CD2-IL-5/rac2–/– eosinophils. In situ release of EPO was determined using OPD as the substrate in samples of 2.5 × 104 freshly prepared eosinophils from CD2-IL-5 and CD2-IL-5/rac2–/– mice (n = 8–11). Cells were stimulated for 30 min in the presence of 3 µm A23187. * p < 0.05, ** p < 0.01.
Marked Eosinophilia in Blood of Rac2–/– C57Bl/6 Mice
To determine the effects of Rac2 gene deletion on circulating eosinophil numbers, we compared the blood eosinophil levels of normal wild-type C57Bl/6 mice (not CD2-IL-5 Tg, which express high levels of eosinophils in both CD2-IL-5 and CD2-IL-5/rac2–/– strains) and rac2–/– mice on the same background. Shown in figure 4 is a representative experiment using 6 C57Bl/6 and 4 rac2–/– C57Bl/6 mice. Irrespective of the locus of blood sampling, each experiment had a statistically significant increase in blood eosinophil numbers in rac2–/– mice. In the representative experiment (fig. 4), there were 4-fold more eosinophils in the peripheral blood of rac2–/– mice compared with wild-type mice (n = 3 experiments). This accumulation of eosinophils may be related to an inability of cells to exit the circulation due to deficits in cell motility.
Eosinophilia in peripheral blood of rac2–/– C57Bl/6 mice. Discombe’s staining of peripheral blood eosinophils was performed on 4–6 mice/group in each experiment; a representative result from 3 experiments is shown. The total number of mice used in 3 independent experiments with different blood sample sources was 18 wild-type and 14 rac2–/– mice. These experiments could not be combined into a single graph, since blood was collected from different parts of the body (femoral, retroorbital), which leads to different total eosinophil numbers in wild-type mice [51]. Wild type refers to C57Bl/6 mice (not IL-5 Tg), rac2–/– refers to rac2–/– mice on the C57Bl/6 background. * p < 0.05.
Eosinophilia in peripheral blood of rac2–/– C57Bl/6 mice. Discombe’s staining of peripheral blood eosinophils was performed on 4–6 mice/group in each experiment; a representative result from 3 experiments is shown. The total number of mice used in 3 independent experiments with different blood sample sources was 18 wild-type and 14 rac2–/– mice. These experiments could not be combined into a single graph, since blood was collected from different parts of the body (femoral, retroorbital), which leads to different total eosinophil numbers in wild-type mice [51]. Wild type refers to C57Bl/6 mice (not IL-5 Tg), rac2–/– refers to rac2–/– mice on the C57Bl/6 background. * p < 0.05.
To explore this possibility, eosinophil levels in the jejunum, a tissue where eosinophils are readily detectable, were analyzed from wild-type control C57Bl/6 and rac2–/– C57Bl/6 mice. There was a trend towards decreased tissue eosinophil levels in the jejunum of rac2–/– mice, although this did not reach statistical significance (data not shown). This is not surprising since our previously published in vitro findings showed that rac2–/– eosinophils showed a statistically significant decrease, but not total abolition of migration [28]. Presumably, there may be compensatory mechanisms for eosinophil tissue migration in vivo. Furthermore, as the amount of eosinophils in the blood compartment is lower than in the tissue, and as eosinophils live for prolonged periods of time in tissue compared with blood, any disturbance of the transition of eosinophils from blood to tissue would be more evident in the blood rather than in the tissue. Therefore, experiments were carried out to analyze the effects of Rac2 on F-actin formation and shape change in eosinophils.
Altered Actin Polymerization in CD2-IL-5/Rac2–/– Eosinophils
We have previously shown decreased transmigration in response to eotaxin-2 in CD2-IL-5/rac2–/– eosinophils [28]. Thus, we tested the hypothesis that this is a fundamental phenomenon for actin polymerization occurring in both CCR3- and non-CCR3-induced stimuli. Eosinophils were treated with eotaxin-2 (CCL24) and PAF, and their F-actin content was assessed by flow cytometry. Actin polymerization, measured as F-actin content, in response to eotaxin-2, was decreased between 29 and 128% when compared with CD2-IL-5 eosinophils (average of 4 experiments is 68% decrease; p = 0.01, paired t test; fig. 5a). This decrease was seen over 2 logs of eotaxin-2 stimulation (1–100 ng/ml, data not shown). Similarly, the mean decrease for PAF-induced responses was 49% (n = 3; p = 0.02, paired t test; fig. 5b). A similar decrease was also seen with 100 nM PAF (data not shown). These data suggest that actin polymerization in eosinophils in response to chemoattractants is dependent on Rac2.
F-Actin formation in CD2-IL-5/rac2–/– eosinophils. Eosinophils were stimulated with eotaxin-2 (10 ng/ml) (a) and PAF (10 nM) (b) for indicated periods of time, and relative F-actin fluorescence determined by binding of NBD-phallacidin and flow cytometry. Representative figure from 3 experiments.
F-Actin formation in CD2-IL-5/rac2–/– eosinophils. Eosinophils were stimulated with eotaxin-2 (10 ng/ml) (a) and PAF (10 nM) (b) for indicated periods of time, and relative F-actin fluorescence determined by binding of NBD-phallacidin and flow cytometry. Representative figure from 3 experiments.
Shape Change Is Dependent on Rac2 in Eosinophils
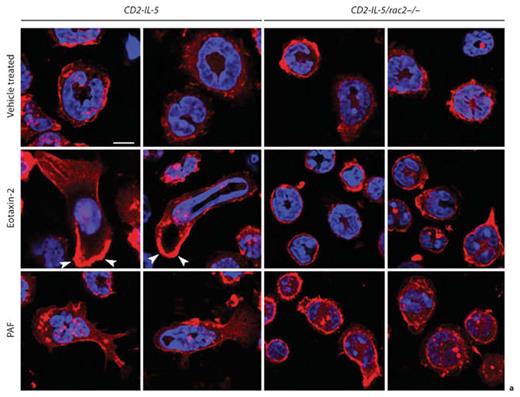
We next examined Rac2-dependent eosinophil shape change by confocal microscopic analysis.Control vehicle-treated CD2-IL-5/rac2–/– eosinophils appeared slightly more rounded than CD2-IL-5 cells and possessed a prominent cortical F-actin region (fig. 6a). Following eotaxin-2 stimulation, CD2-IL-5 eosinophils demonstrated a profound shape change in the form of morphological stretching accompanied by cell polarization, with an actin-rich leading edge and evidence of actin stress fibers in the tail end of cells. Nuclei were also stretched in some CD2-IL-5 cells, indicating significant flexibility in the nuclear structure. By counting round and stretched cells in 10 high-powered fields per condition, cell stretching was evident in 2% of the total counted cell population in resting CD2-IL-5 eosinophils (1/51 cells), while eotaxin stimulation increased the proportion of morphologically stretched cells to 40% (38/95 cells; p < 0.0001, Fisher’s exact test, 2-sided).
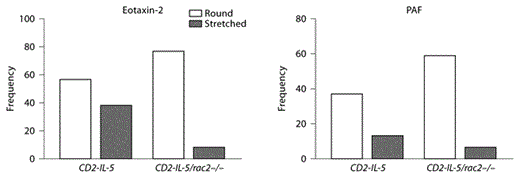
Shape changes are altered in CD2-IL-5/rac2–/– eosinophils during chemoattractant stimulation. a Cells were stained with fluorescent F-actin-binding rhodamine-phalloidin (red), and counterstained with the nuclear stain DAPI (blue), following stimulation for 10 s with eotaxin-2 (10 ng/ml) or PAF (10 nM). Arrowheads indicate intense deposition of F-actin at the leading edges of migrating cells. Scale bar = 10 µM. b Frequency plots showing morphologically round and stretched cell shapes in eosinophils stimulated with eotaxin and PAF. Quantitation of cells was done from 7 to 11 high-powered fields taken from a 63× objective.
Shape changes are altered in CD2-IL-5/rac2–/– eosinophils during chemoattractant stimulation. a Cells were stained with fluorescent F-actin-binding rhodamine-phalloidin (red), and counterstained with the nuclear stain DAPI (blue), following stimulation for 10 s with eotaxin-2 (10 ng/ml) or PAF (10 nM). Arrowheads indicate intense deposition of F-actin at the leading edges of migrating cells. Scale bar = 10 µM. b Frequency plots showing morphologically round and stretched cell shapes in eosinophils stimulated with eotaxin and PAF. Quantitation of cells was done from 7 to 11 high-powered fields taken from a 63× objective.
CD2-IL-5/rac2–/– eosinophils showed low levels of morphological stretching in resting cells (6%, 4/71 cells). However, CD2-IL-5/rac2–/– eosinophils exhibited less shape change in response to eotaxin compared with CD2-IL-5 eosinophils (fig. 6b). Morphological stretching in CD2-IL-5/rac2–/– eosinophils in response to eotaxin was 9% (8/85 cells) compared with 40% in CD2-IL-5 eosinophils (difference 31%; 95% confidence interval 17.8–43.4%; p < 0.0001, Fisher’s exact test, 2-sided).
When cells were stimulated with PAF, CD2-IL-5 eosinophils displayed significant shape change, while CD2-IL-5/rac2–/– eosinophils showed decreased shape change. PAF was less potent than eotaxin-2 at inducing shape changes. Cell stretching in CD2-IL-5 eosinophils in response to PAF was 26% (13/50 cells), and in CD2-IL-5/rac2–/– eosinophils 9% (6/65 cells; difference 17%, 95% confidence interval 3.0–30.5%; p =0.022, Fisher’s exact test, 2-sided). Nuclear stretching was also not evident in PAF-activated cells as that in eotaxin-stimulated cells.
Discussion
We report a specific role for Rac2 in eosinophil O2– release, exocytosis, F-actin formation, and shape changes in response to chemoattractants. We show that Rac2 is partially required for O2– release and exocytosis, and that it has an active role in F-actin formation and shape changes in response to both CCR3-dependent and -independent chemoattractant stimulation. Defects in shape changes in CD2-IL-5/rac2–/– eosinophils during treatment by eotaxin-2 and PAF correlated with deficiencies in F-actin formation in eosinophils.
These findings are similar to rac2–/– BMNs, which have deficient O2– release and F-actin formation leading to cell motility [14,16], as well as ablated exocytosis [19]. However, there were some significant differences in CD2-IL-5/rac2–/– eosinophils, which had reduced deficiency in O2– generation (26% reduction compared with CD2-IL-5 eosinophils) compared with that of BMNs (60% reduction). Since Rac1 and RhoG can also activate the NADPH oxidase complex at the plasma or phagosomal membrane [9,38], these are proposed to act as preferential GTPases for oxidase activation in eosinophils. PMA-induced O2– release involves protein kinase C activation, which phosphorylates guanine exchange factors, leading to guanosine diphosphate (GDP)/GTP exchange on Rac1 and Rac2 [35]. In contrast, O2– release from BMNs showed a greater dependency on Rac2 than eosinophils, which is reflected in the inability of rac2–/– mice to eliminate fungal infections [16], and reduced lung injury in an immune complex-mediated model of acute lung injury [39].
An interesting observation in this study is that eosinophils from CD2-IL-5 mice generated a small fraction (4–5%) of the amount of O2– that human eosinophils produce in response to the same dose of PMA [27]. While mouse eosinophils generated approximately 0.7 nmol O2–/106 cells, human eosinophils produced approximately 16 nmol O2–/106 cells. Mouse eosinophils from schistosome-induced hepatic granulomas have been shown to generate negligible O2–, while peritoneal lavage cells containing eosinophils produced measurable O2– release, although both of these were mixed cell populations [37]. Mouse models of airway hyperresponsiveness have shown that eosinophil numbers and their activation do not always correlate with the induction of allergic airway disease using ovalbumin [40,41]. Instead, intense recruitment and activation of eosinophils to the lungs is required to induce an inflammatory phenotype, such as that seen in IL-5/eotaxin double transgenic mice [42]. It is possible that mouse eosinophils are not as readily activated in mouse models of airway hyperresponsiveness and produce relatively less oxidants, and this may present a hindrance when studying these models in comparison with allergic airway inflammation and asthma in humans. However, it is also conceivable that overexpression of IL-5 may have altered the responsiveness of splenic eosinophils to stimuli in these experiments. Moreover, human eosinophils from atopic patients showed enhanced O2– production [24,25]. These caveats have to be taken into consideration with the fact that it is not possible to obtain sufficient numbers of eosinophils from normal healthy human donors, or wild-type control mice, in order to carry out these assays for degranulation and superoxide release. Thus, in mouse studies, IL-5 transgenic mice provide the only source of mouse eosinophils that can be used for measuring eosinophil effector functions in vitro. Our studies on eosinophils from IL-5 transgenic mice are not unprecedented for analyzing effector functions of these cells [28,43].
Taken together, our findings from CD2-IL-5/rac2–/– eosinophils suggest that other Rho GTPases such as Rac1 regulate the release of O2– from eosinophils. However, Rac1 knockout mice are embryonic lethal, and although conditional mutants are available, it is technically demanding to generate sufficient numbers of eosinophils from Rac1 conditional mutants. Experiments on eosinophil respiratory burst using cells from Rac1 conditional mutants are beyond the scope of our study at present.
There have been scant and conflicting reports on mouse eosinophil degranulation; while some suggest that this is undetectable [41,44], 2 studies showed in vitro release of MBP in response to PMA [45], and EPO release in response to interferon-γ [43]. In contrast, there is substantial evidence of EPO release and degranulation from airway eosinophils in vivo in several mouse models of asthma [42,45,46], and human eosinophils readily degranulate following stimulation with 1–10 µM calcium ionophore A23187, leading to cytolytic release of granules [33,47,48]. Mouse eosinophils in our study were found to release slightly less EPO than reported for human eosinophils (approx. 35 vs. 55% for human) using A23187 [33]. Our observations indicate that Rac2 contributes to Ca2+-dependent degranulation responses in eosinophils. There are a number of pathways that could activate Rac2 in Ca2+-dependent degranulation, including protein kinase C, leading to guanine exchange factor phosphorylation and subsequent GDP/GTP exchange on Rac2. This is postulated to lead to Rac2-mediated actin remodeling as a prerequisite for granule movement and exocytosis. Our inability to test degranulation responses to physiological stimuli in mouse eosinophils has made it impossible to evaluate the role of Rac2 in response to receptor stimulation. This is a known limitation of studying mouse eosinophil functions in vitro [42].
In response to 2 distinct chemoattractants (eotaxin-2 and PAF), eosinophils displayed an essential role for Rac2 in F-actin formation at early stages of motility that was not substituted by normal expression of Rac1. These chemoattractants act on 2 distinct 7-transmembrane G-protein-coupled receptors, CCR3 and PAF receptors, to induce chemotaxis in eosinophils [28]. CD2-IL-5/rac2–/– eosinophils displayed a rounded phenotype that was maintained in the presence or absence of stimulation by chemoattractants. These cells lacked polarization, actin capping at the leading edge, and nuclear stretching. The pathway(s) that leads from Rac2 to shape change and degranulation is not well understood, but likely involves the activation of actin nucleation factors such as Arp2/3 and cofilin, which are required for actin assembly leading to shape changes and increased cell motility in leukocytes [49]. What remains to be determined is to examine how Rac2 can be discriminated from other Rho GTPases by receptor signaling leading to actin filament formation, such as Cdc42 which also acts on the actin nucleating factor Arp2/3 through interaction with the CRIB domain-containing N-WASP protein [50]. The expression of cofilin, Arp2/3 and their associated interacting proteins has not yet been determined in eosinophils. The findings of deficient F-actin formation and shape change in eosinophils in the absence of Rac2 may explain the observed increase in peripheral blood eosinophilia in Rac2-deficient mice. However, it remains possible that the mechanism of baseline peripheral blood eosinophilia includes alteration of eosinophil production or apoptosis; these alternative explanations will be formally explored in future studies.
In conclusion, our study indicates a specific role for Rac2 in eosinophil exocytosis, F-actin formation, and shape change in response to chemoattractants. Rac2 contributes to maximal O2– generation from eosinophils, although the quantity and rate of O2– generation is comparatively smaller than that of human eosinophils. These findings have implications for the role of Rac2 in eosinophil function and provide insight into the specific mechanisms by which eosinophils migrate to tissues, leading to cell activation and the release of cytotoxic mediators. This study also sheds light on the mechanism of activation of mouse eosinophils, an overlooked area in asthma and allergy research.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research, The Lung Association of Alberta and Northwest Territories, Canada Foundation for Innovation, and NIH AI045898. The authors thank Laura Kindinger, Amy Hajek and Victoria Summey for their technical assistance. We are grateful to Dr. David A. Williams, Children’s Hospital, Boston, Mass., USA, for providing the Rac2-deficient mice for this study and reviewing the manuscript.






![Fig. 4. Eosinophilia in peripheral blood of rac2–/– C57Bl/6 mice. Discombe’s staining of peripheral blood eosinophils was performed on 4–6 mice/group in each experiment; a representative result from 3 experiments is shown. The total number of mice used in 3 independent experiments with different blood sample sources was 18 wild-type and 14 rac2–/– mice. These experiments could not be combined into a single graph, since blood was collected from different parts of the body (femoral, retroorbital), which leads to different total eosinophil numbers in wild-type mice [51]. Wild type refers to C57Bl/6 mice (not IL-5 Tg), rac2–/– refers to rac2–/– mice on the C57Bl/6 background. * p < 0.05.](https://karger.silverchair-cdn.com/karger/content_public/journal/iaa/156/2/10.1159_000322597/2/m_000322597_f04.gif?Expires=1716386785&Signature=KAolYGURbb4gGaC3FwY~xCjFAByVh-Yr5AfXaQm~XUtWgSV966vojGpJgdXiHVPRVEAcaKzU8Cjg8xFBZjumF3iQC3zSZCSkebS1Lie7t3cCIJZcnXDCivWmbkGbWdZ1~Os2lsKnxtc9zMkijcCdwHSvoGxyDXnrn8fiFjfzx9vNY1wxcMMfkjco6zyr90pTAdMDrNNXro5TEwxD3jOTTlbKN~7yOVB-G~SVFu4iKg2zZ5L0s6ycCirewVrChIf9QnmEBjvarCqJfVKrBVceC14F-t7WJ5uW5r9EqkP1sa0k1gEi4OpXgHdZiDQD1w4p9xQN2Ypnfu-zklpNHzLZhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)